Equipment: A test tube, A boiling tube, A heating pad, Bunsen burner, Wooden splint, A bottle of acid, A piece of metal, Safety glasses
Hypothesis: I think that when the acid and metal react with each other I will expect to see bubbles erupting and when I put a match stick it will create popping noises.
Method:
1. Wear Safety glasses so nothing gets into your eyes and light the Bunsen Burner.
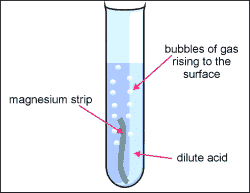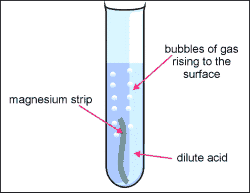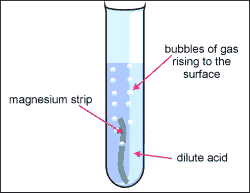
2. Be cautious and put the acid inside of the test tube, you only need 2mL.
3. grab your sample of metal and put it into your test tube containing and acid.
4. Grab the two test tubes and hold them together for a few minutes, allowing time for the inverted boiling tube to fill with gas from the acid and metal reacting with each other.
5. When you think the tube is full, you or your lab partner should light a wooden splint.
6. Carefully, but quickly, tilt the boiling tube full of gas upwards and insert the burning splint into the mouth of the test tube. Make sure you don't burn yourselves or spill the acid and metal.
Observations: The first few times we tried it didn't work until we were told to put our finger on top of the tube with the acid and metal and then put the splint in. when we did the experiment the popping noises always changed on how loud it was.
Discussion: This was because the Hydrogen gas combusted.
Conclusion: My hypothesis was was correct, the gas did pop when the splint and the hydrogen gas were close together
Great Blog Trisha, great experiment report.
ReplyDelete